Phase 2: Year 1&2 Objectives/Methods
Objectives
- Assess adherence to mitigation guidance over time & impact of the pandemic on health & well being
- Assess SARS-CoV-2 incidence & prevalence over time and proportion of infections that are asymptomatic
- Assess proportion of SARS-CoV-2 infections among vaccinated and previously infected
- Assess how SARS-CoV-2 incidence & prevalence differ by key demographic, socioeconomic, health, and behavioral factors
Study timeline

Methods
Recruitment
Participants were initially recruited from Phase 1 of the ComPACT Study for the Phase 2 cohort study. A second wave of recruitment occurred at the beginning of year 2 of the cohort study in an effort to further diversify the ongoing cohort. All participants were enrolled via phone prior to the first study visit.
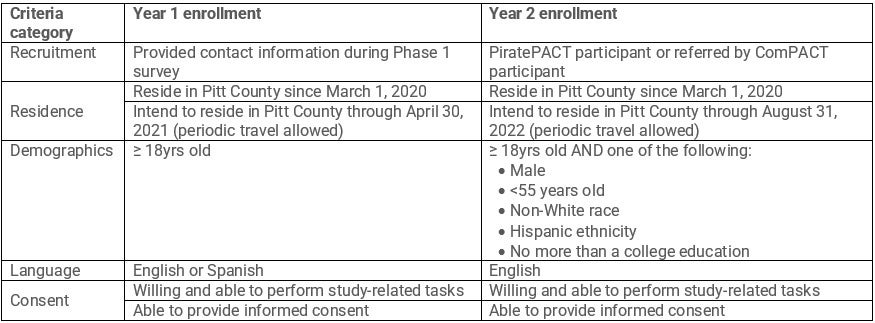
Eligibility criteria for the ComPACT study cohort
Data Collection
There were three main components to data collection for the cohort study: questionnaire, nasal swab, and venipuncture.
Questionnaire
Beginning with the baseline study visit, participants completed online study questionnaires every 2 weeks:
- Annual baseline questionnaire: Administered at the year 1 and year 2 baseline visits and again at the end of the study (August 2022). This survey covered multiple aspects of socio-demographics, food security, physical and mental health (including COVID-19 symptoms and prior testing), COVID-19 risk perceptions, work-related and personal COVID-19 prevention, resilience and emergency preparedness, and some related questions about others in the household.
- Follow-up questionnaire: Administered every two-weeks. This questionnaire covered COVID-19 prevention, some questions on physical health including COVID-19 symptoms and treatment, mental health and wellness (asked every 4 weeks), and household members.
Nasal Swab
Mid-turbinate nasal swabs were self-collected by study participants every two weeks and tested at the North Carolina State Laboratory of Public Health (SLPH). One collection occurred during in-person study visits that coincided with venipuncture (see below). The second collection occurred at home and was mailed directly to SLPH using provided shipping materials. During summer months all nasal swabs were collected at home.
- Mid-turbinate nasal swabs are inserted approximately one inch into each nostril. All participants watched an instructional video prior to the first collection. The first two nasal swabs were directly observed by a health care worker to ensure proper procedures were followed.
- All samples were tested for SARS-CoV-2 using the Taq-PathTM RT-PCR COVID-19 Multiplex.
Venipuncture
Venipuncture for SARS-CoV-2 antibody testing occurred every four weeks (once per month) during an in-person study visit.
- Serum was tested for SARS-CoV-2 antibodies using the Abbot Architect chemiluminescent microparticle immunoassay:
- During Year 1, samples were tested for nucleocapsid antibodies (Immunoglobulin G [IgG]), which develop after natural infection
- During Year 2, samples were tested for both nucleocapsid IgG antibodies and spike IgG antibodies (develop after infection or vaccination).
- Year 1 stored samples were also tested for spike IgG antibodies after the text became available.
- Remaining serum was stored